Cultivation and Procurement of Raw Material Crude Drugs
Securing a Stable Supply of Safe Crude Drugs
Tsumura’s Efforts for Securing Crude Drugs
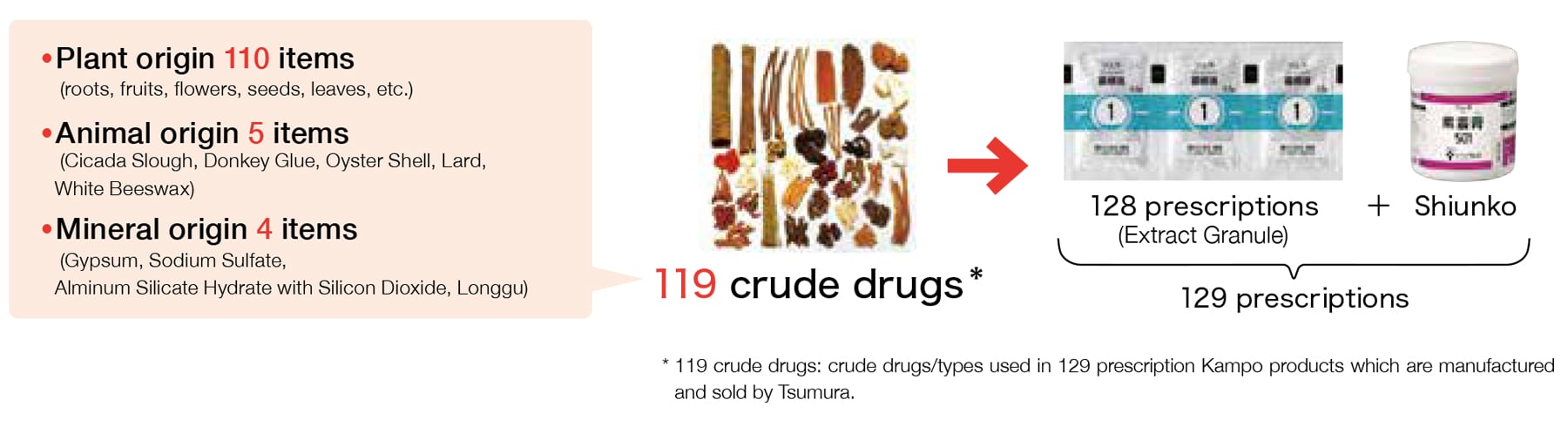
To produce Kampo products, we need to secure high quality raw material crude drugs. They are produced by preparing and processing* medicinal part (roots, stalks, fruits, leaves, etc.) of plants, animals, minerals, etc.
In order to select high quality crude drugs, we conduct comprehensive quality evaluation by investigating a classical textbook (“Honzousho”) on crude drugs, and reconciling the contents with modern science to determine the botanical origin of the crude drug as well as geographical origin. Then we conduct an appearance test, sensory test, internal morphological test, and genetic test as well as physical and chemical test (including component determination).
In addition, we acquire sample crude drugs in advance in an original form without cutting, in principle, and conduct a quality inspection to adequately evaluate the quality of raw material crude drugs. Then, quality tests are performed by lot on the crude drugs that passed the quality inspection. Thus, only quality assured crude drugs are delivered for production.
As described, quality raw material crude drugs we acquired are cut into adequate sizes and used as crude drug pieces, or mixed according to prescription and sent to production process for extract granules to become high quality Kampo products.
- Preparing and processing: Drying, steaming, and processing harvested crude drugs
Management of Crude Drug Production
From the time of leaving the production site to reaching SHENZHEN TSUMURA or Ishioka Center, raw material crude drugs go through many phases such as cultivation, processing, transporting, storage, etc. We utilize crude drug traceability system to keep a record of production history including timing and condition at individual phases. Combining this system with the historical data of manufacturing processes and distribution processes for Kampo products, we now have ability to track and retroact the historical data of the entire processes from the production site of raw material crude drugs to healthcare institutions.
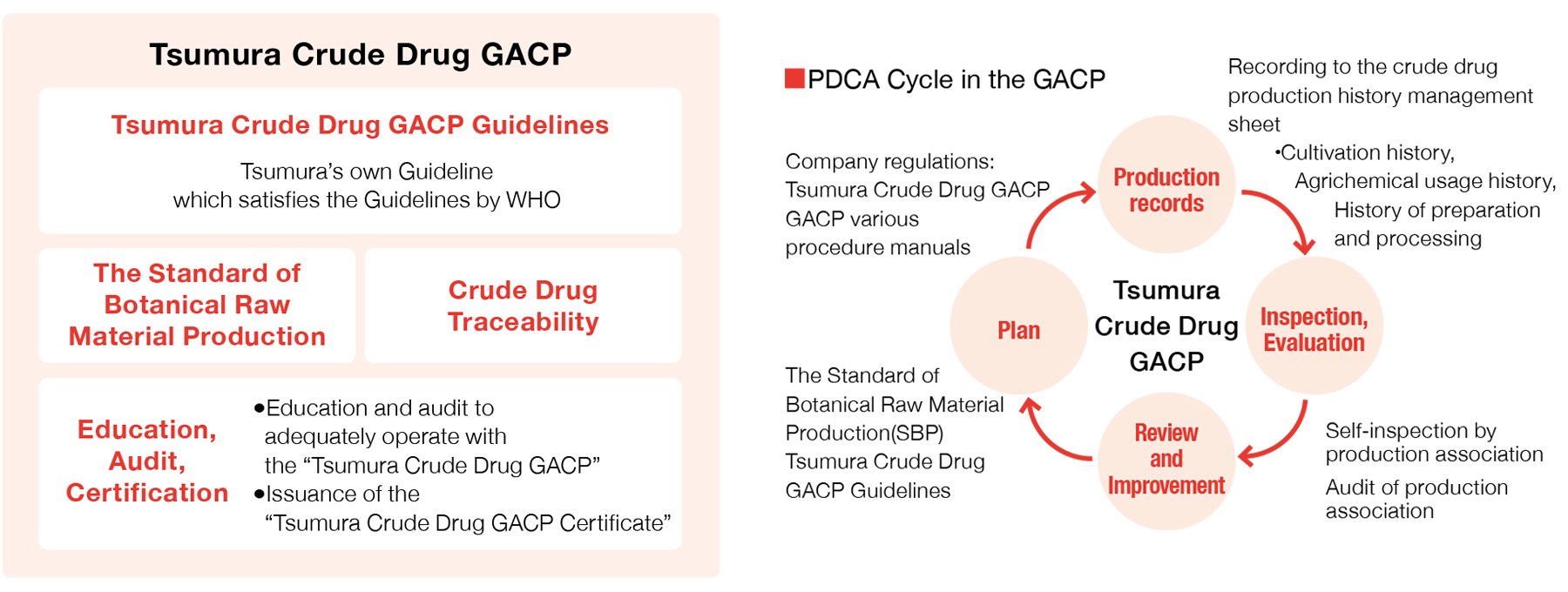
In 2010, we established the TSUMURA & CO. standards for Management of Crude Drugs Production (the Tsumura Good Agricultural and Collection Practice (Tsumura Crude Drug GACP)), which is composed mainly of the “Standard of Botanical Raw Material Production*1 ,” “Crude Drug Traceability,” and “Tsumura Crude Drug GACP*2 Guidelines” with addition of “Education, Audit, Certification” system by referencing the GAP*3 certification system, which is a process management system for general agricultural produces. We will continue to operate the PDCA cycle adequately with the Tsumura Crude Drug GACP by improving and reinforcing our crude drug production management system to realize stable procurement of safe crude drugs.
- ※1Standard of Botanical Raw Material Production: A document summarizing crude drug harvesting methods agreed between Tsumura and individual grower organizations for each crude drug to enable us to obtain raw material crude drugs with the quality Tsumura requires, methods of various post-harvest activities such as preparation and processing methods, storage and transportation methods, permitted agrichemicals for use during cultivation, etc.
- ※2GACP: Good Agricultural and Collection Practice
- ※3GAP: Good Agricultural Practice
Management of Wild Medicinal Plants
As for wild medicinal plants we purchase from China, we identify harvesting sites, harvesting timing, and representatives of harvesting work. To assure quality and safety, quality tests and inspections including residual agrichemical tests are conducted for all raw material crude drugs in the same manner as for cultivated crude drugs.
Development of Cultivation Technology and Application in Actual Production
We are conducting researches to achieve 100% cultivation of plant-based crude drugs through development of cultivation technology. In China, we conduct joint researches in cultivation of crude drugs with relevant research organizations. In Japan, researches are conducted mostly in Hokkaido for improving yield of crude drugs and stabilizing quality, in addition to the researches on cultivating wild medicinal plants. With the advancement of cultivation, the number of crude drugs we need to rely only on wild plants has been decreasing. Thus, we will continue to promote cultivation, and ensure stable acquisition of safe crude drugs.
Management of Crude Drug Production in China
In China, we are able to retroact individual crude drug farmers to confirm any necessary information using a well-established system that enables the representatives*1 identified by the production site companies*2 to collect information related to cultivation in the group they represent.
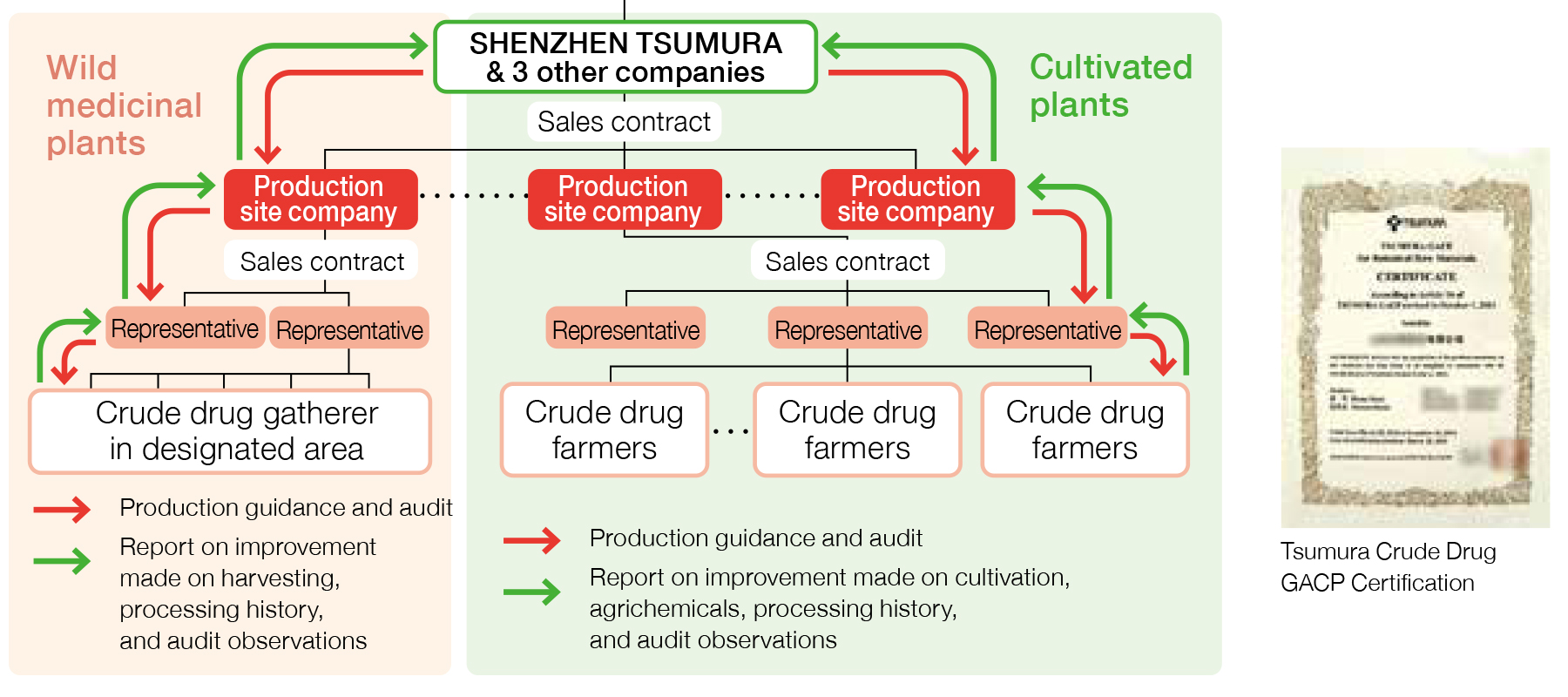
- ※1Production site company: Within the scheme of crude drug procurement in China, production site companies serve to supply raw material crude drugs to Tsumura’s crude drug procurement-related companies (SHENZHEN TSUMURA, and three other companies). They are generally located at the production site, and manage the cultivation of crude drugs as well as purchase crude drugs from crude drug farmers and conduct preparation and processing.
- ※2Representatives: Organizers of crude drug farmers. They are in charge of managing crude drug farmers, gathering information, providing education and organizing shipping cargos.
Management of Crude Drug Farmers in China
Crude drug farmers in China are managed through production site companies as they provide education and guidance to the farmers through their representatives in accordance with the Crude Drug GACP. We are able to identify individual farmers from which we purchased each crude drug through the production site companies. Crude drug famers are identified either before starting cultivation of crude drugs or at the time of purchasing crude drugs.
Managing Production Site Companies in China
We manage production site companies in China by conducting audits to all of them based on the Crude Drug GACP. These audits are conducted to assess production site companies’ ability to manage cultivation and processing environment as Tsumura requires. We purchase raw material crude drugs only from the production site companies that had passed the audit and received the Crude Drug GACP Certification. The certification is valid for three years. Thus we conduct an audit every three years to renew the certification. In case when a production site company does not pass the audit, the certification will not be renewed, and we do not purchase from the company. When we conduct the audit, we select some crude drug farmers from the name list of the representative to check if they are cultivating as instructed.
