Process of Kampo Medicines to Reach Patients
-
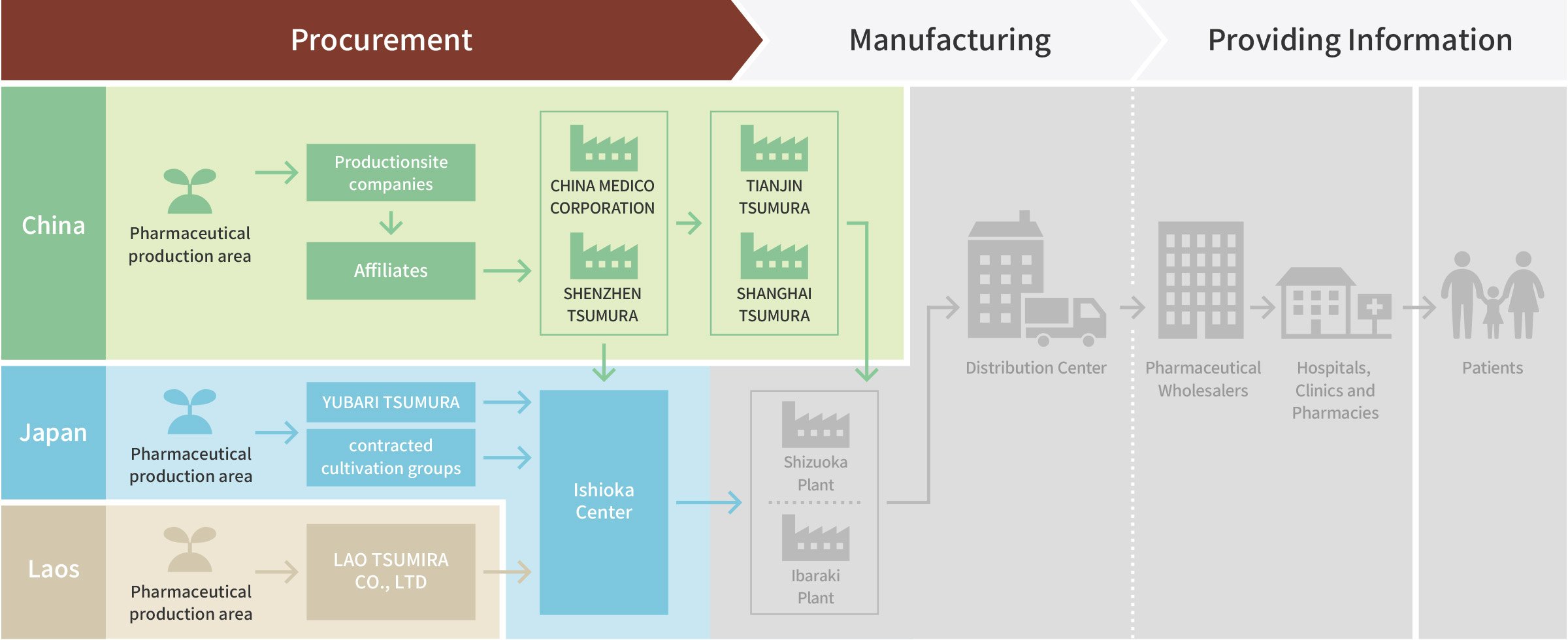
Procurement
STEP
1
Cultivation of Raw Material Crude Drugs
A large portion of raw material crude drugs is cultivated,and we control cultivation methods and agrichemicals usage as it is required to meet a certain quality level for raw materials of pharmaceutical products. When wild medical plants are used, we select plants of the same quality level.
-

The People’s Republic of China (China) -

Japan -

The Lao People's Democratic Republic (Laos)
STEP
2
Procurement, Preparation and Processing,and Storage of Raw Material Crude Drugs
Raw material crude drugs procured in individual sites are scrutinized to ensure no foreign objects or low quality materials are mixed in. Only those materials that meet our standard are processed and stored in a low-temperature storage to maintain the quality
-
The People’s Republic of China (China)
-

Production sitecompanies
(approximately 130 companies)
-

Affiliates

SHENZHEN TSUMURA MEDICINE CO., LTD.(Shenzhen, China) -
-
Japan
Contracted cultivation groups Six key sites
-

YUBARI TSUMURA
(Yubari, Hokkaido, Japan)
-

Other contracted cultivation groups

YUBARI TSUMURA -
-
The Lao People's Democratic Republic (Laos)
-

LAO TSUMURA
(Saravan Province, Laos)

LAO TSUMURA 
Processing plant -
STEP
3
Quality
TestSelection and Processing, Storage
Quality tests are conducted on agrochemical residues, micro-bacteria, heavy metals, etc., and only those raw material crude drugs that satisfy the quality standard set by the Tsumura Group are supplied to individual plants for manufacturing of Kampo products.

-
SHENZHEN TSUMURA MEDICINE CO., LTD.(Shenzhen, China)
-


To Ishioka Center and SHANGHAI TSUMURA PHARMACEUTICALS CO., LTD.
-
-
Ishioka Center(Ishioka, Ibaraki Prefecture, Japan)
-


To Shizuoka Plant and Ibaraki Plant
-
Related page
-
-
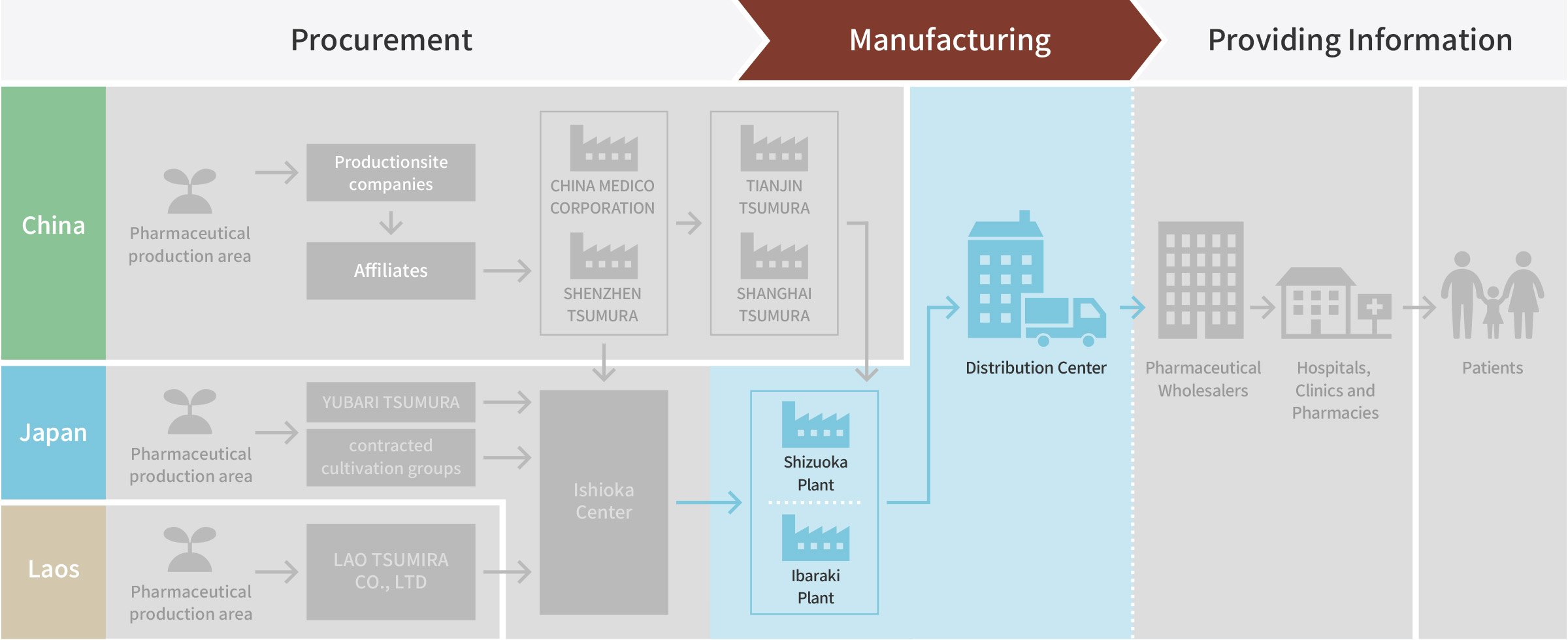
Manufacturing
STEP
4
Process
InspectionCutting
Raw material crude drugs stored in their original form are delivered to a plant. During cutting process, they are cut into an adequate size for extraction using a cutting machine to fit each shape.

Crude drugs after cutting -
-

SHANGHAI TSUMURA PHARMACEUTICALS CO., LTD.
(Shanghai, China)

-
-
-

Shizuoka Plant
(Fujieda, Shizuoka Prefecture, Japan)

-
-
-

Ibaraki Plant
(Ami-machi, Inashiki-gun, Ibaraki Prefecture, Japan)

-
STEP
5
Process
InspectionWeighing and Mixing
After cutting, raw material crude drugs are weighed in accordance with each prescribed composition. Then, they are placed in a large mixing container, and sent to extraction process after checking the mass again.
-

Weighing -

Mixing
STEP
6
Process
InspectionExtraction, Separation, and Concentration
Extraction is performed using our original device made to employ the same traditional extraction methods. Extracted essences are filtered by centrifuge, concentrated in short time at a low temperature using a device with the minimum effect on the Kampo ingredients.
-

Extraction -

Separation and Concentration
STEP
7
Quality
TestDrying
Concentrated liquid is transferred to a large spray dryer. To avoid heat effect, concentrated liquid is sprayed from the top of the dryer and instantly dried and cooled to become extract powder
- 上海津村のエキス粉末は、静岡工場・茨城工場に 移送し、造粒工程に送ります。

Drying(Extract powder) STEP
8
Quality
TestGranulation
Extract powder is mixed with a diluting agent and directly compressed to mold with a tablet press. Then, the molds are crushed and sized into extract granules. This dry granulation process will not change the ingredients in any way.
-

Granulation -

Molded extract powder -

Extract granules
STEP
9
Quality
TestPackaging and Labeling
Quality confirmed extract granules are sent to the packaging and labeling process. They are sheet wrapped by the unit of daily quantity, and packaged in a bundle of seven sheets. Then, they are shipped as Tsumura’s prescription Kampo product extract granules.
STEP
10
Shipping, Storage, and Delivery
Products shipped out from plants are stored at a distribution center, and then delivered to pharmaceutical wholesalers.
Shipping
Storage and Delivery
-
-

East Japan Distribution Center
(Kuki, Saitama Prefecture)

-
-
-

West Japan Distribution Center
(Ono, Hyogo Prefecture)

-
Related page
-
-
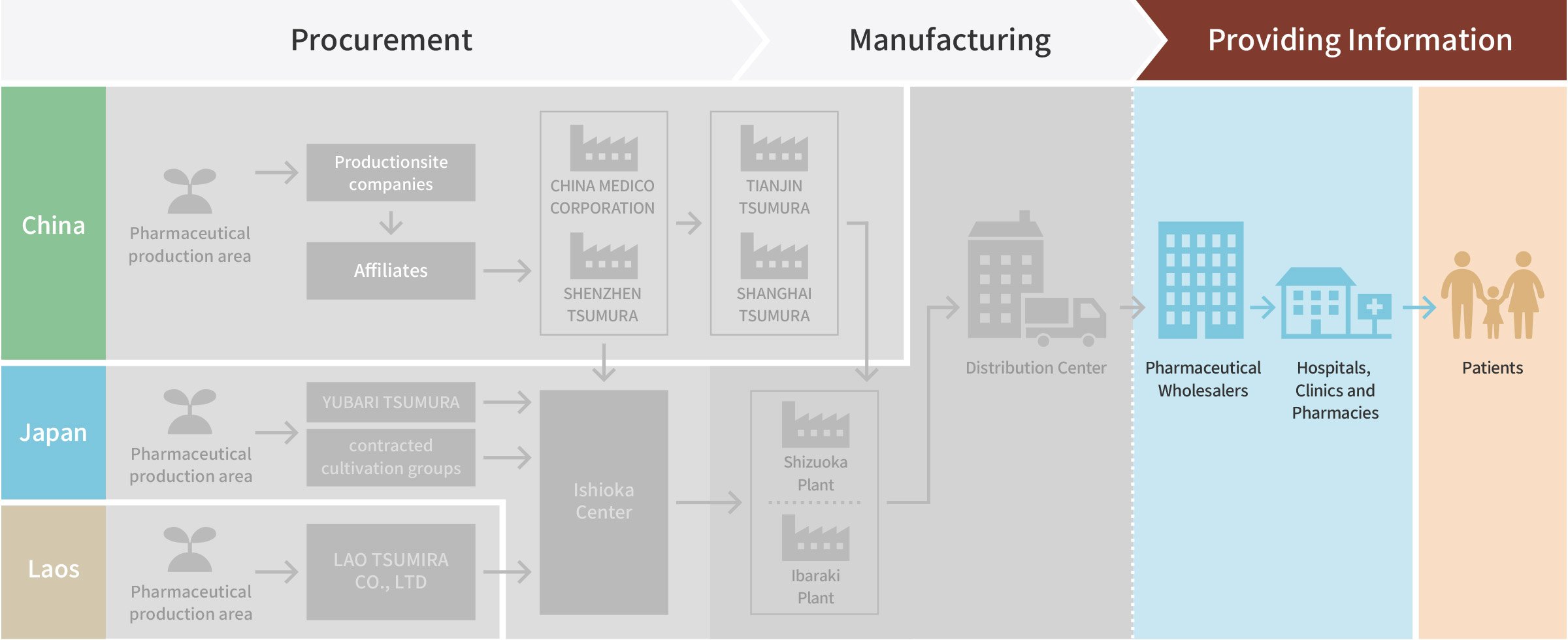
Providing Information
STEP
11
Pharmaceutical Wholesalers
When products are delivered to wholesalers, marketing specialists (MS*) sell them to medical institutions and pharmacies. At that time, they provide necessary information for appropriate usage. When distributing pharmaceutical products, it is important to deliver information with the products.
- MS : Marketing Specialist

STEP
12
Hospitals, Clinics and Pharmacies
Medical representatives (MR*) provide information on Kampo medicines by visiting healthcare institutions and holding explanation meetings in the medical staff office.
- MR:Medical Representatives

STEP
13
Patient
Kampo medicines are dispensed by pharmacist according to the prescriptions provided by clinical physicians, and reach the patients.

Related page










