Quality control, Production, and Distribution
Enhance the Quality Control System
Tsumura Quality Management System
Under the quality policy, we have established the Tsumura Quality Management System to further improve the quality assurance system and promote initiatives to prioritize quality. This Quality Management System is a comprehensive system for the entire Tsumura Group to participate in, and the involvement of management members has been even more clarified. Also, the system is formulated in a way that we can adequately respond to globalization (including PIC/S* response) and revisions of laws and regulations.
Quality Policy
The Tsumura Group has formulated a quality policy consisting of the following principles in line with its aim to be a value-creation company that contributes to people’s health through its Kampo business:
- Providing a stable supply of high-quality, reliable products
- Complying with laws and regulations related to pharmaceuticals
- Listening to customer feedback and striving to continually improve the quality of our products
- Realizing stable procurement of safe crude drugs
- Offering appropriate training to all our executives and employees, and cultivating human resources distinguished by a high degree of awareness
- Adequately allocating our management resources to help achieve these principles
- PIC/S: The abbreviation of the “Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme”, which is a framework for advancing internationalization of standards such as GMP.
Quality and Safety Management of Raw Material Crude Drugs
Raw material crude drugs procured in various sites in China are mainly gathered in SHENZHEN TSUMURA, while those procured in Japan and other countries excluding China are sent to Ishioka Center. The equivalent quality tests are conducted in both sites, and only the raw material crude drugs that meet the Tsumura inhouse quality standard are supplied to the plants where Kampo products are manufactured. Particularly for safety related quality items including residual agrichemicals, microbes, heavy metals, we are developing own testing methods at the Product Research and Analysis Center, and conducting quality tests and evaluating quality. Furthermore, we have introduced the analysis technology developed at the Product Research and Analysis Center to SHENZHEN TSUMURA, and quality tests are conducted there for raw material crude drugs originated in China.

Quality and Safety Management of Products
The present quality standards of Kampo preparations were formulated in accordance with notification from the Ministry of Health and Welfare (at that time) in 1980 (Notification No. 804) and 1985 (Notification 2 No.120).
Based on these standards, many items are tested including component quantitative test with mechanical analysis. In addition, as our own standard, quality tests are conducted by the Product Research and Analysis Center in terms of safety against residual agrichemicals and microbes.

Quality Tests
Residual Agrichemicals
For raw material crude drugs and Kampo products, we conduct analysis of residual agrichemicals stipulated in pharmacopeia of relevant countries as well as all agrichemicals used in cultivation of crude drugs in Japan, China, Laos, etc., and agrichemicals that are prohibited to use in those countries. As crude drugs and Kampo products contain many different ingredients, it is necessary to have technology to efficiently extract agrichemicals only to analyze a very small portion of residual agrichemicals. We have developed such technology internally, and are conducting tests and inspections. In addition, we are developing technology for testing unexpected residual agrichemicals due to residues in soil, drift (scattering of agrichemicals sprayed on other agricultural produces), etc. Such analysis results are provided to cultivation fields as feedback to be utilized for further improvement of agrichemical management.
Inspection of Residual Agrichemicals
We have formulated Tsumura In-house Standard for agrichemicals of the following conditions, and are conductingresidual agrichemical inspections for approximately 200 kinds of agrichemicals.
- Agrichemicals for which the Japanese Pharmacopeia restricts residual quantities (2 agrichemicals)
- All agrichemicals used in cultivation of crude drugs that Tsumura purchases
- Agrichemicals that Tsumura considers necessary to control based on own risk assessment
Number of agrichemicals to be inspected are updated in accordance with the three conditions indicated above.
| Crude Drugs | Kampo Products | ||
|---|---|---|---|
| TSUMURA | In-house standards | All lots and all items |
Monitor all items* |
Microbes
We inspect all lots of extract powders and Kampo products in accordance with the microbial tests listed in the Japanese Pharmacopeia. While various components in the test samples inhibit the detection of microbes during the test, we conduct highly reliable tests by utilizing technology developed internally.
Heavy Metals and Arsenic
For raw material crude drugs and extract powders, we conduct inspections in accordance with the standards and methods provided in the Japanese Pharmacopeia. In addition, we have developed a method to analyze four hazardous elements (cadmium, lead, mercury, arsenic), using ICP-MS* individually and with high sensitivity. We are controlling such elements, in order to supply Kampo products with a sense of security by detecting detailed risks.
- ICP-MS: Inductively Coupled Plasma – Mass Spectrometer
Aflatoxin
Aflatoxin is a type of mycotoxins produced by certain fungi, and adversely affects people and animals. We have developed own analysis method for aflatoxin, and are controlling the risk of aflatoxin contamination on Kampo products.
Radioactive Substances
With the accident of nuclear power plant occurred with the Great East Japan Earthquake in March 2011, we have faced a new issue of test control system for radioactive substances. In response to the issue, the Ministry of Health, Labour and Welfare issued a notification on December 13th, and the Federation of Pharmaceutical Manufacturers’ Associations of Japan formulated the “guideline for determination of radioactive materials in crude drugs and the like.” Based on this guideline, we confirm the safety of raw material crude drugs, water used in manufacturing, and Kampo products to control quality.
Enhancement of Production Capacity
Sales volume of prescription Kampo products has been increasing steadily. Based on a long-term demand forecast, we are planning to increase production capacity for extract powder, granules, and products as well as securing staff and developing human resources. In order to maintain a stable supply system, we will maximize our current production capacity at the three sites, Shizuoka, Ibaraki and Shanghai, while gradually expanding facilities. Specifically, we will make efforts for introducing new manufacturing technology such as robotic technology, and for propelling manufacturing by saving manpower and energy, as well as improving the basic capacity of current facilities.
Saving Manpower and Energy with Robotic Technology
As a way of reforming production system, we are automating manufacturing process by introducing new manufacturing technologies such as robotics.
Robots with in-house specification developed by incorporating technologies of various fields have been introduced to the facilities for transportation in manufacturing process, feeding raw materials, packaging, etc. Introduction of robotics has enabled us to implement 24-hour continuous production, and shifted manufacturing work to monitoring of manufacturing. This has contributed to the improvement of productivity while reducing workload of employees and enhancing hygiene control.

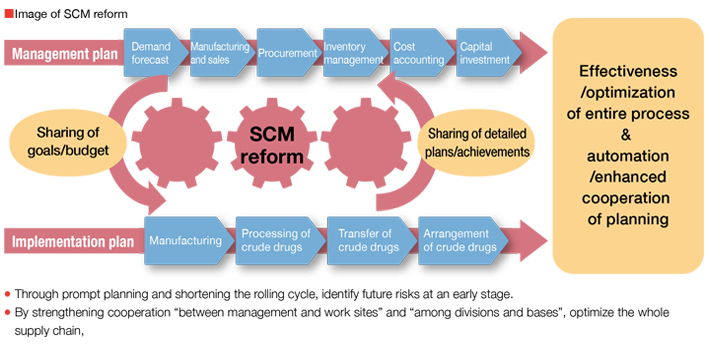
Optimization of the Group Supply Chain
Kampo products are produced after going through many processes starting at procurement of raw material crude drugs including cultivation. We have engaged in the reform of work process by understanding the total supply chain including individual processes and between those processes within the groups in Kampo and crude drug business as well as procurement sites of crude drugs outside of our company group and product sales. For further improvement, we will execute managementOptimization of the Group Supply Chain incorporating the concept of supply chain management (SCM*), a reform method for realizing further advancing efficiency and optimization. Through project activities across the Group, we will realize an SCM reform and build a foundation for responding to changes in business environment promptly.
- SCM: We wish to build an SCM system to automate and speed up processes by coordinating sales plan, production plan, raw material crude drug cultivation, arrangements making, procurement, processing and transporting, and inventory plan starting with demand forecast.